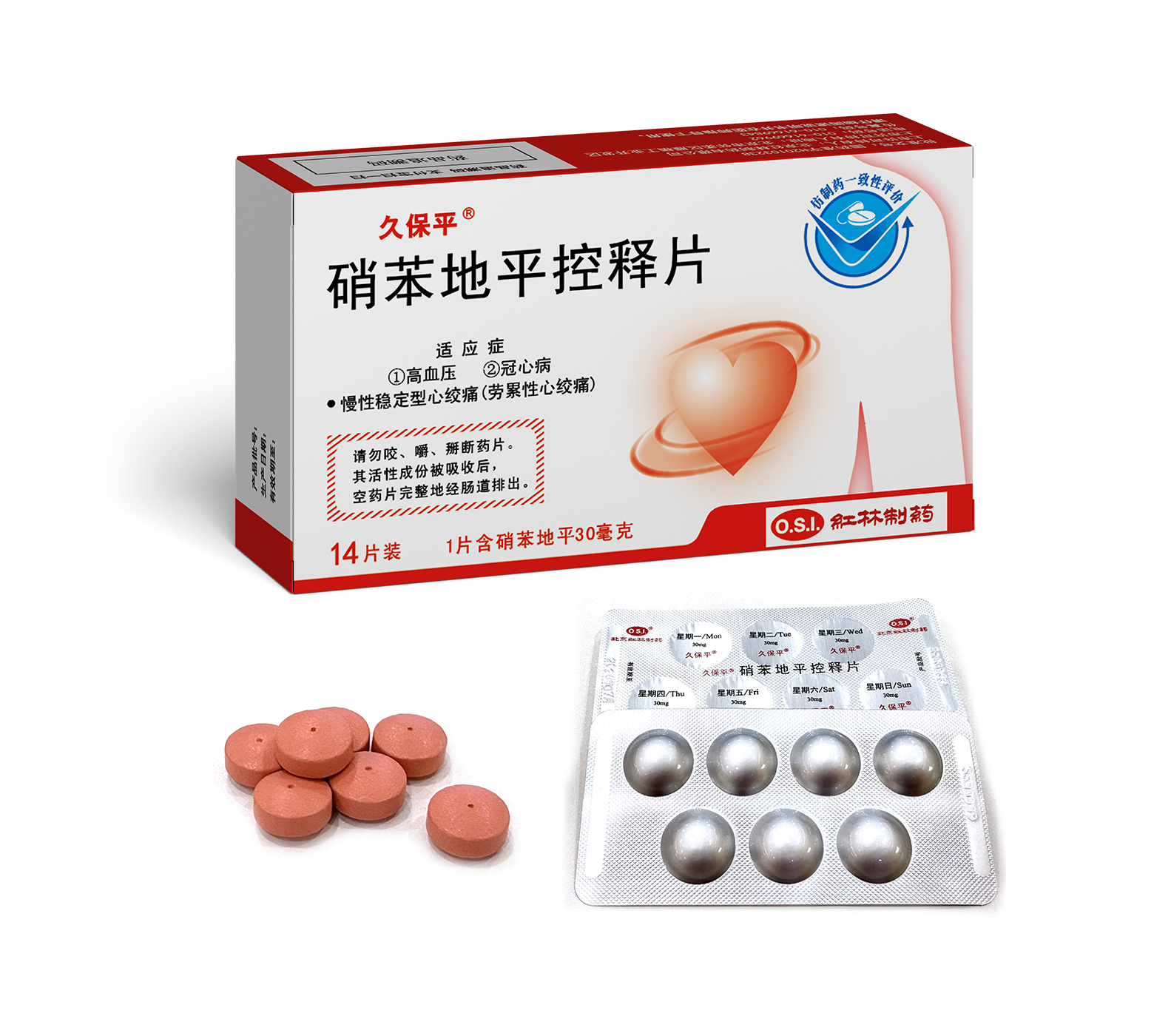
1. Vasospastic Angina
2. Chronic Stable Angina (Classical Effort-Associated Angina)
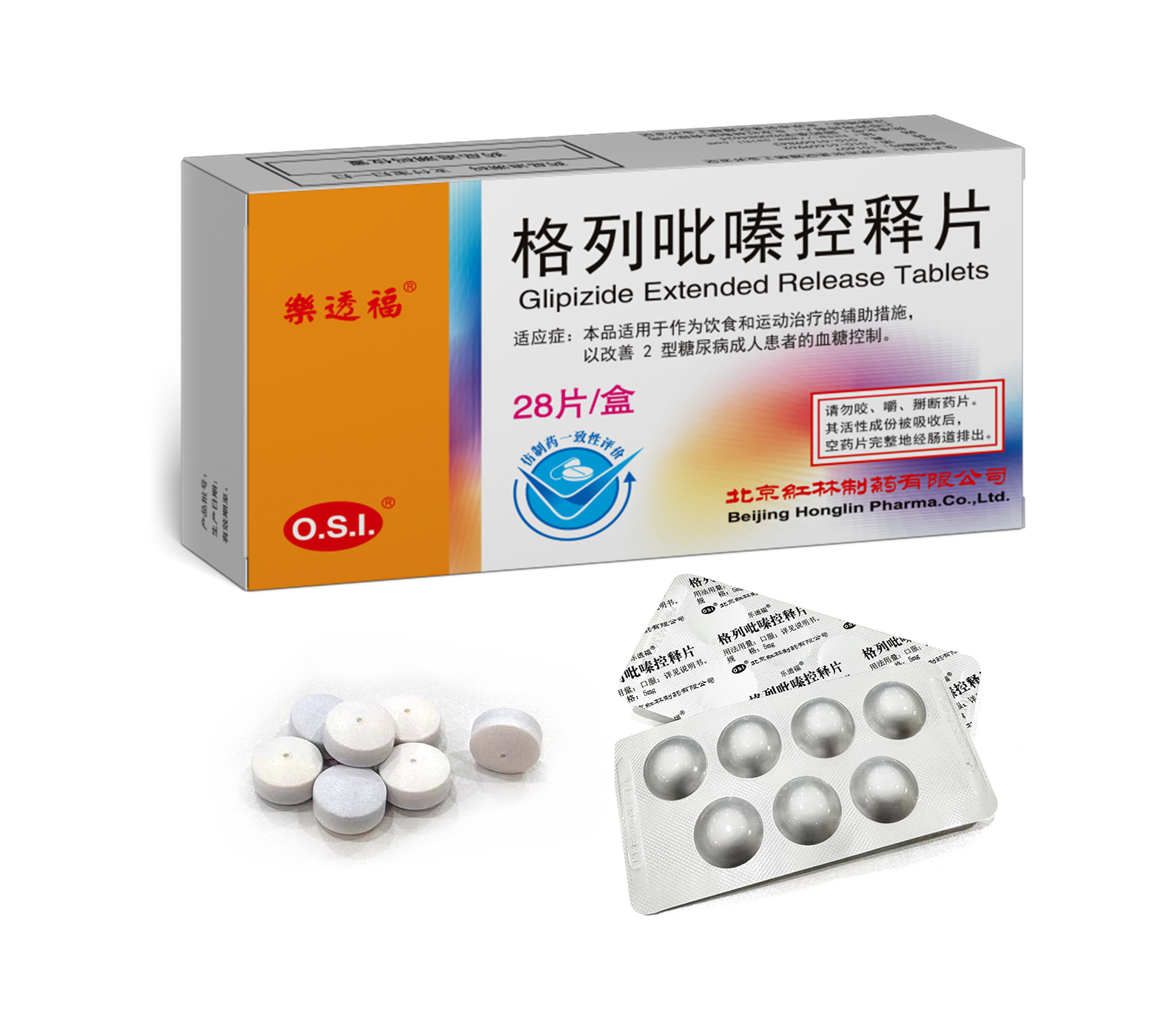
This product is applied to treat hyperglycemia and related symptoms in patients with type 2 diabetes on the basis of controlled diet and exercise.
For alleviating moderate pains, such as arthralgia, neuralgia, myalgia, migraine, headache, dysmenorrheal, toothache;
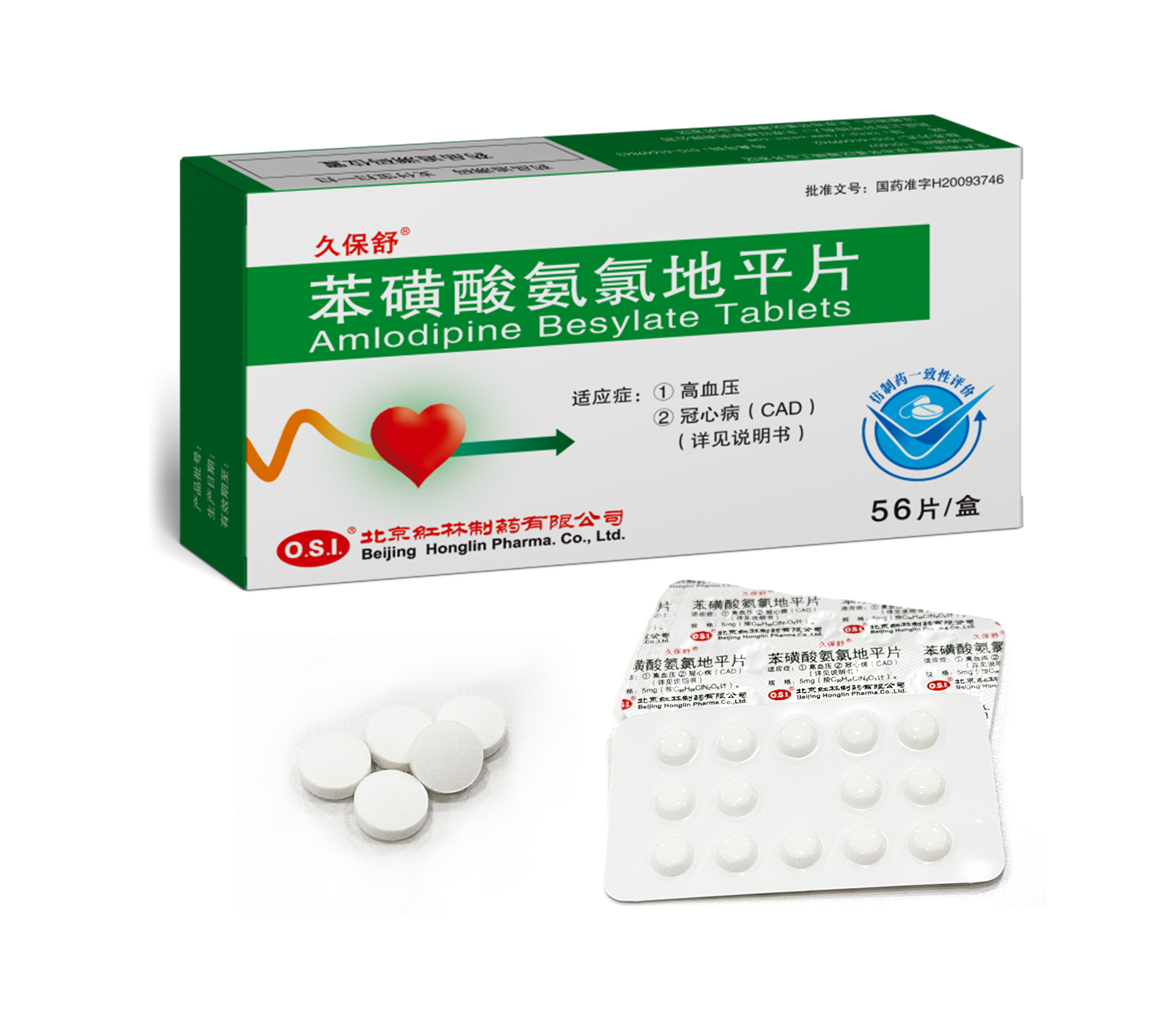
1. Hypertension
2. Coronary artery disease “CAD” (Details in the Users manuals)
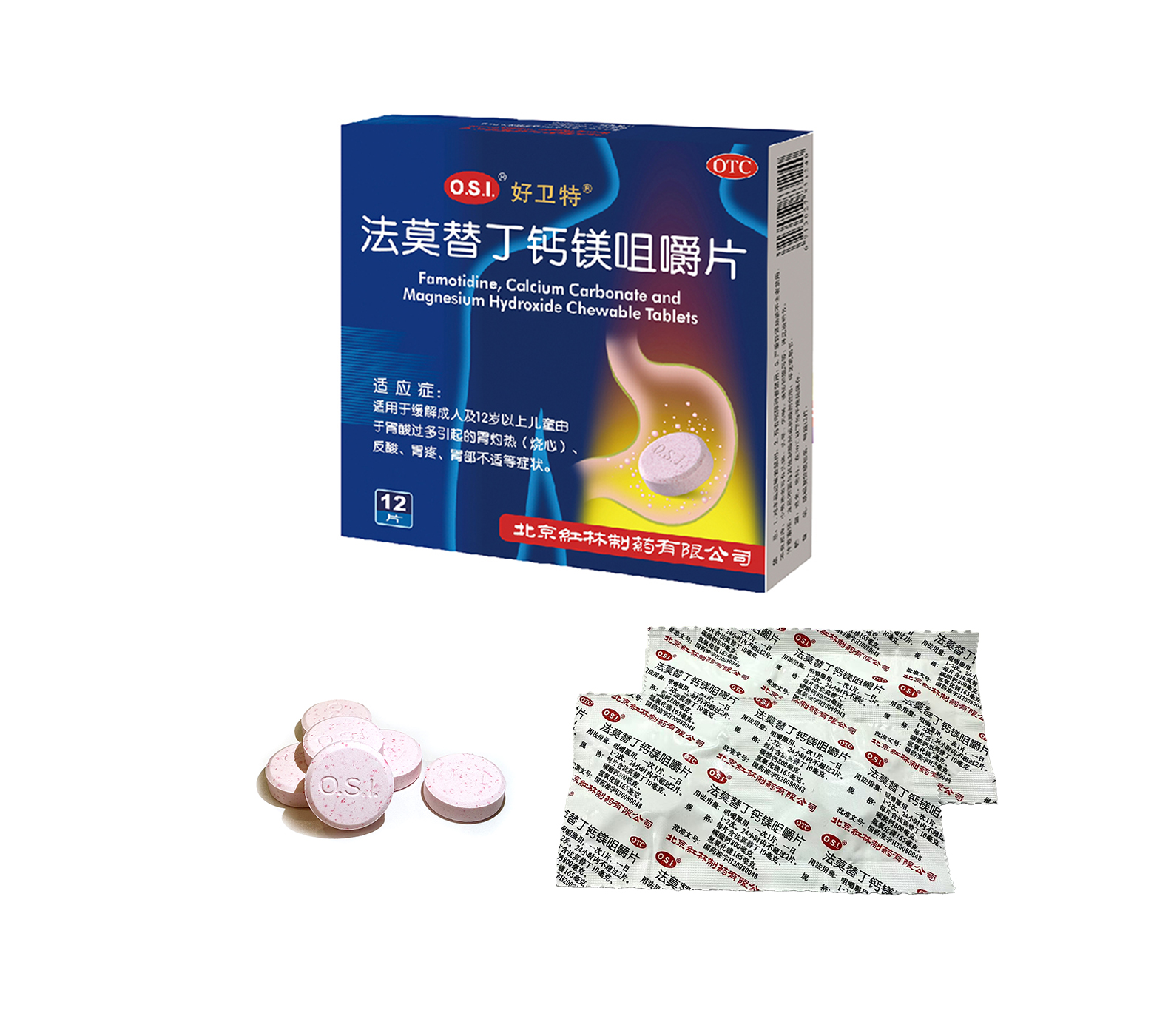
Relief of pyrosis (heartburn), sour regurgitation, stomach ache, stomach
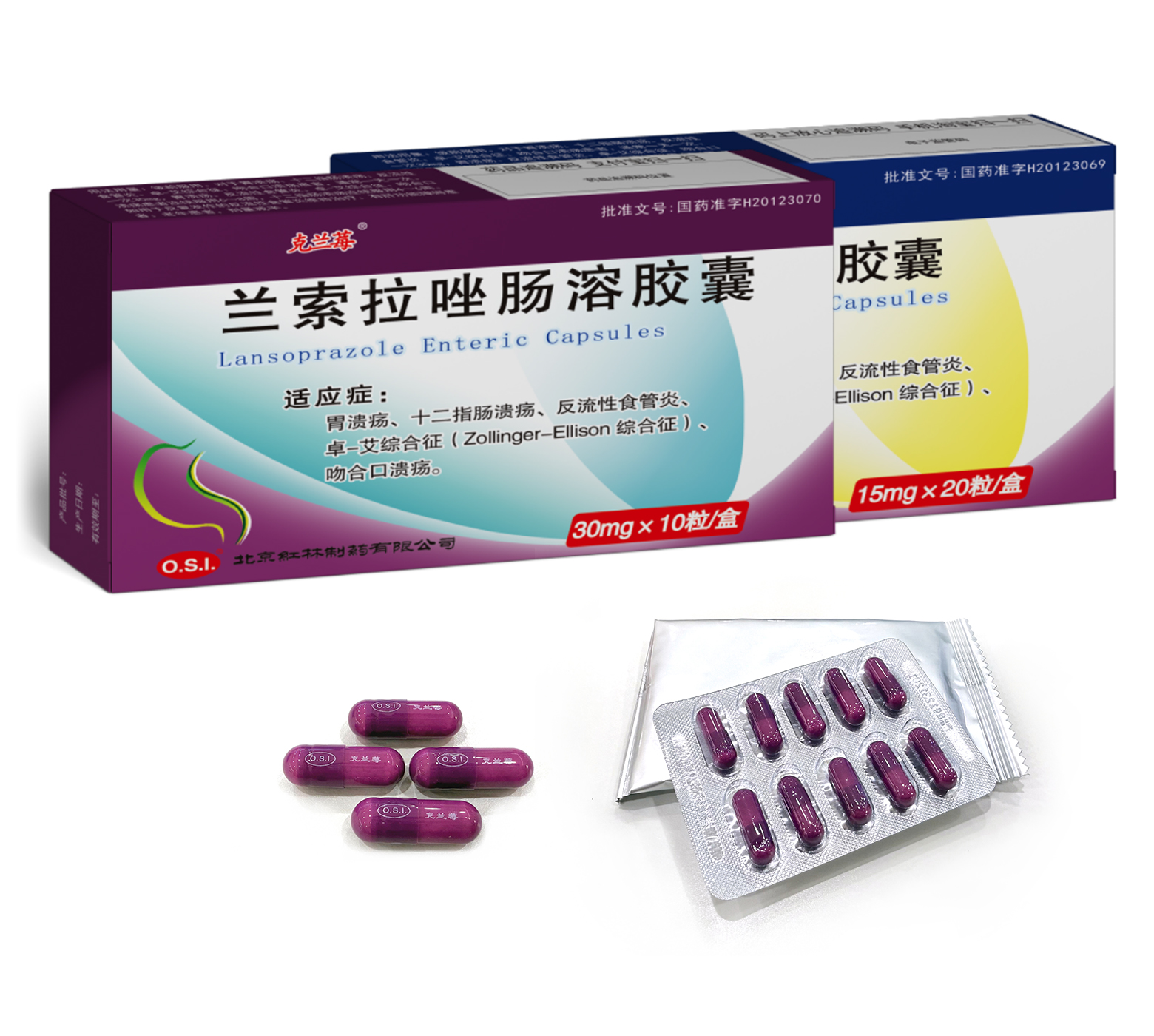
Gastric ulcer, duodenal ulcer, reflux esophagitis, Zollinger-Ellison Syndrome and marginal ulcer.
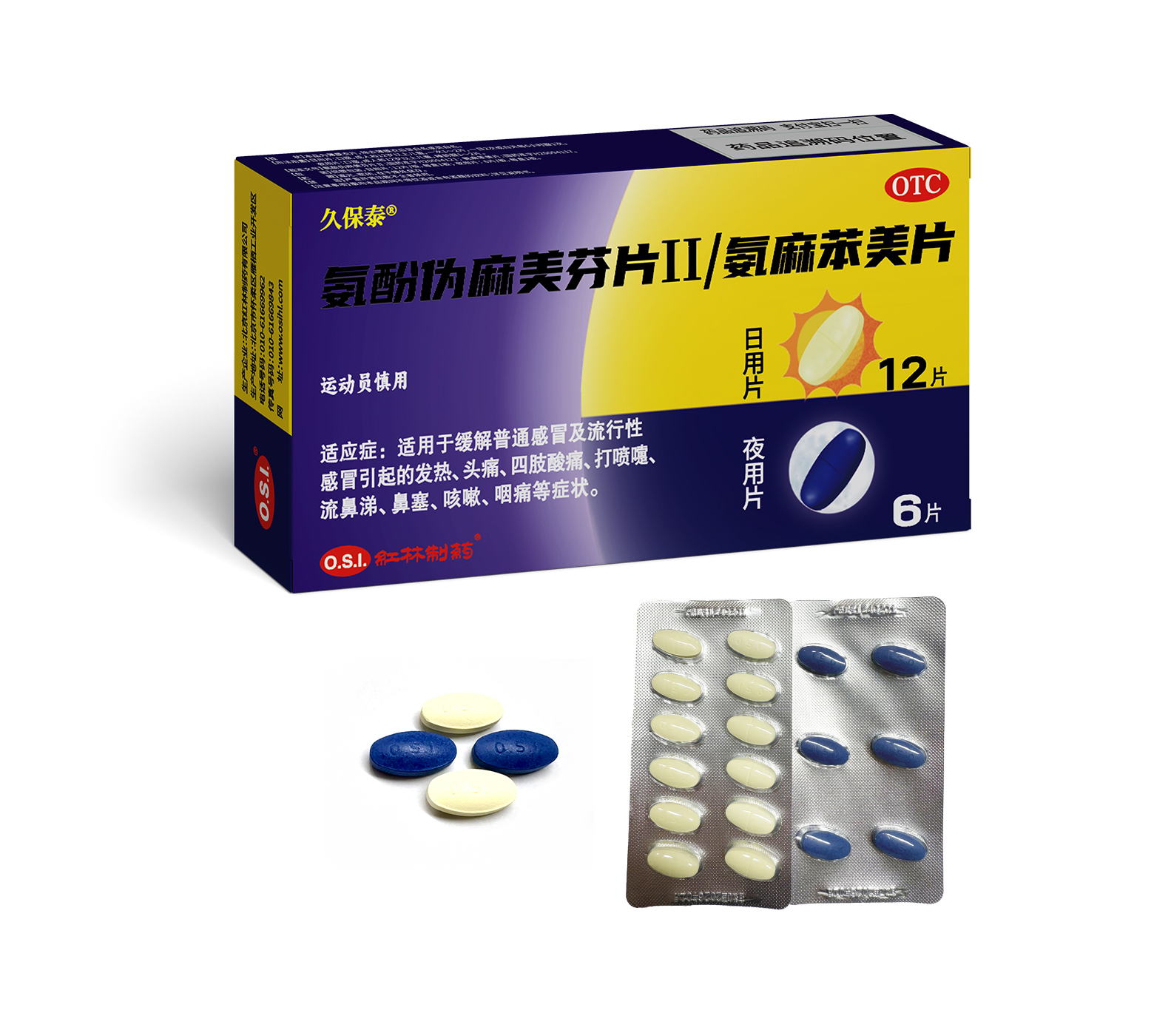
These products are applicable for the relief of fever, headache,limb pain, sneeze, rhinorrhea, nasal obstruction, cough, pharyngalgia
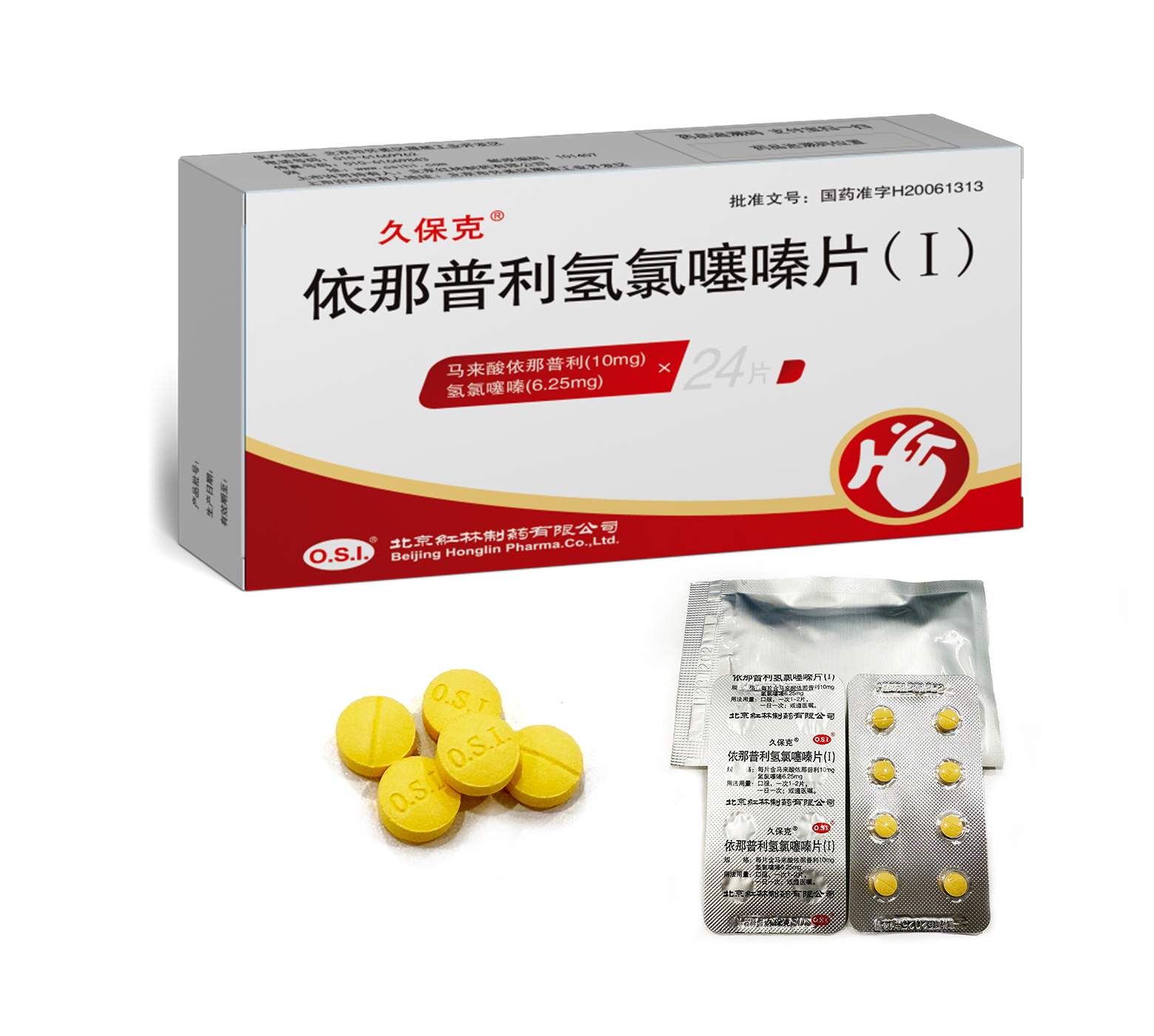
This product is only applicable for the treatment of patients whose hypertension cannot be effectively controlled by single drug treatment
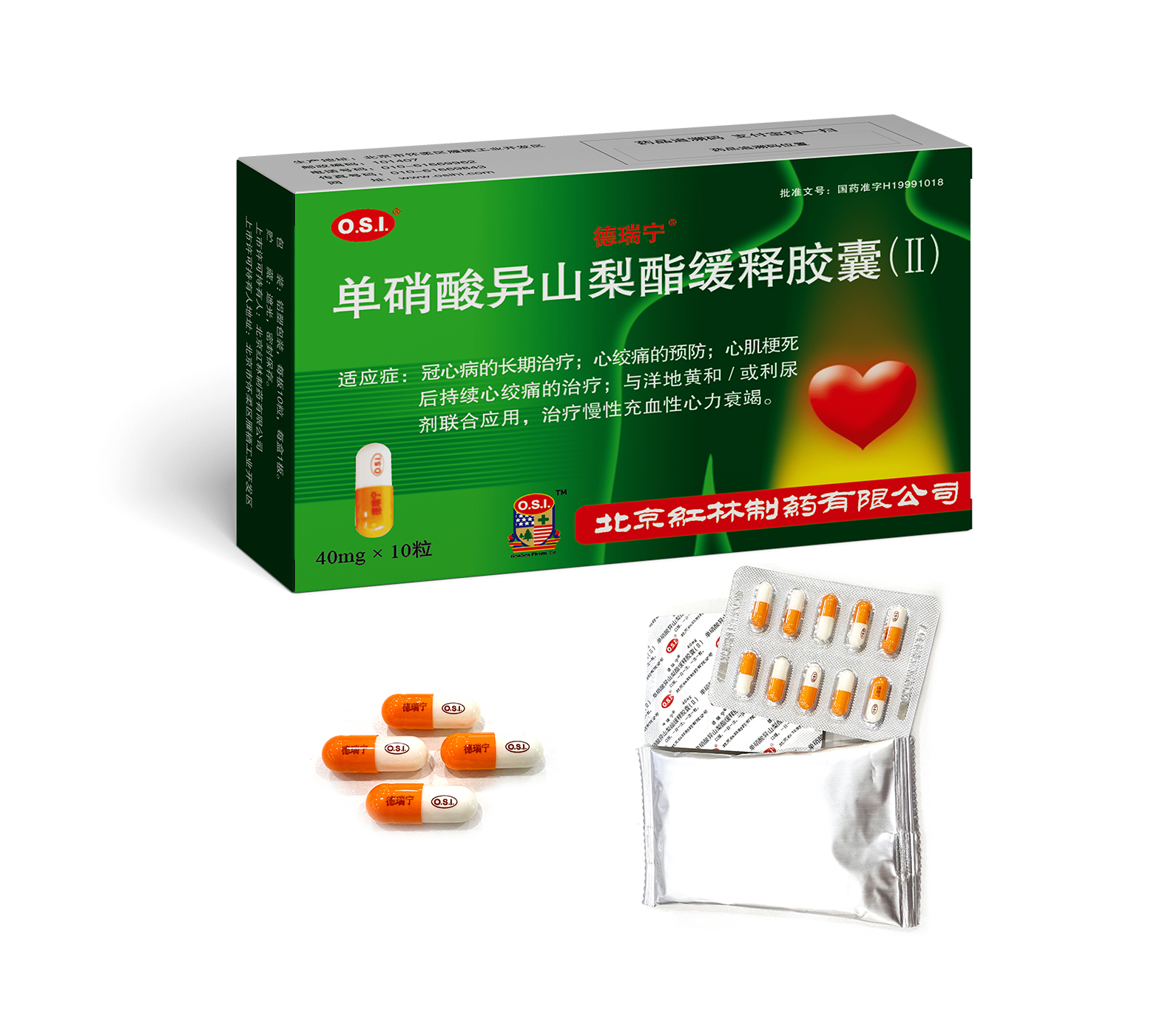
Long-term treatment of coronary heart disease; prevention of angina;treatment of continuous angina after myocardial infarction;
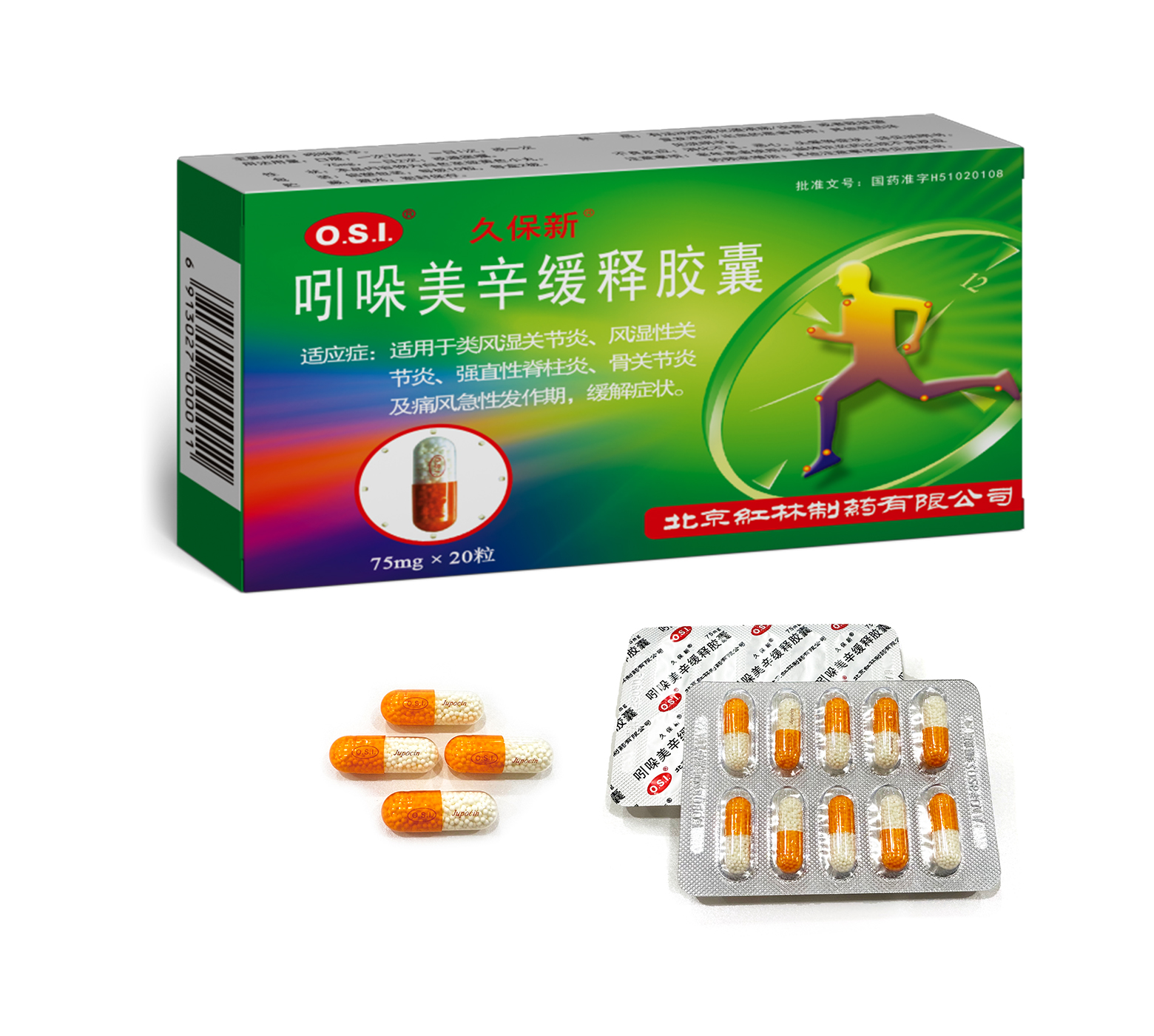
This product is for relieving symptoms of rheumatoid arthritis, rheumatic arthritis, ankylosing spondylitis, osteoarthritis and acute gout arthritis.
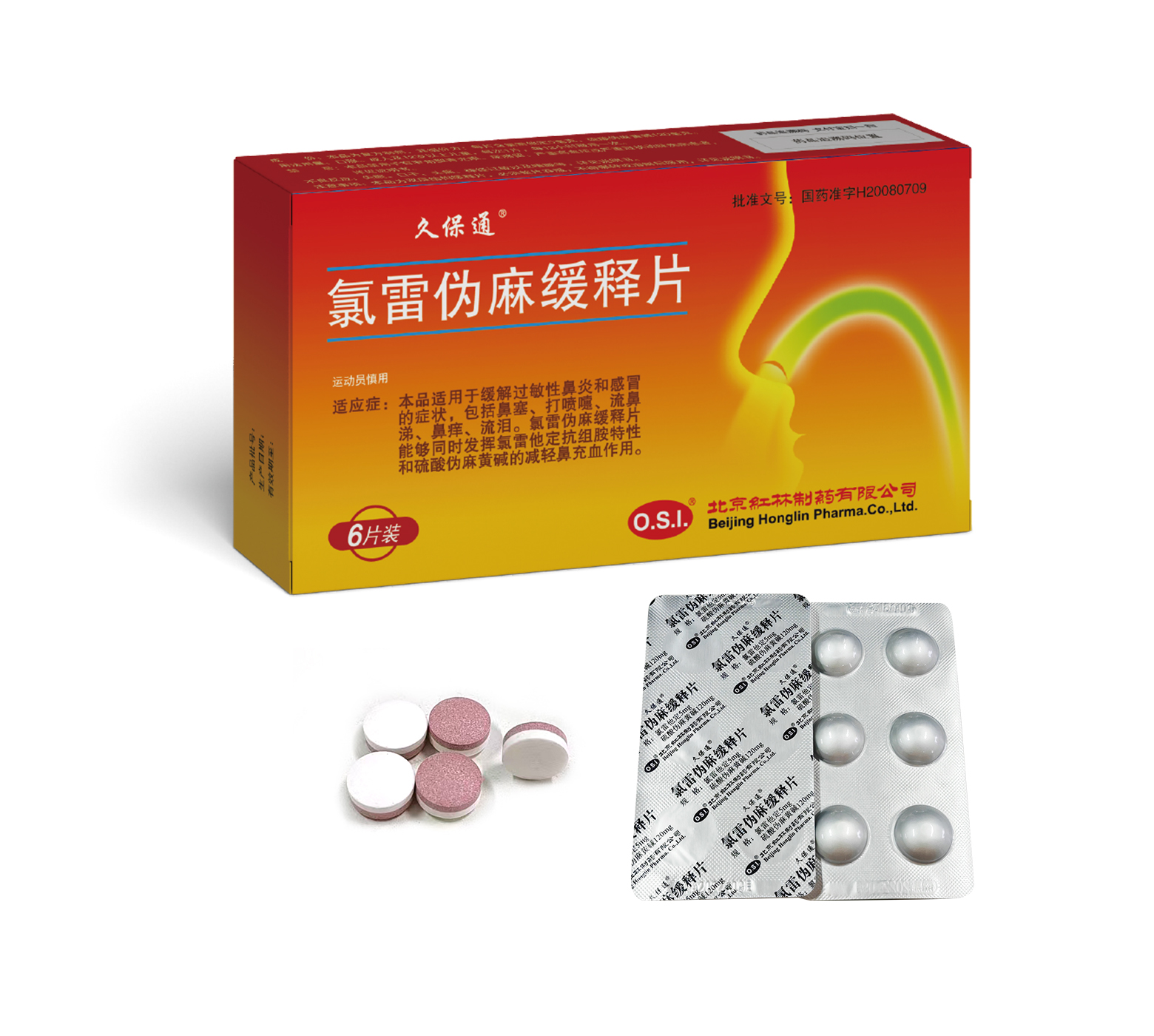
This product is indicated for the relief of allergic rhinitis and cold symptoms, including nasal congestion, sneeze, rhinorrhea, rhinocnesmus and lacrimation.

Treatment of adult depression.

Nifedipine Controlled-release Tablets
Nifedipine
1. Vasospastic Angina
2. Chronic Stable Angina (Classical Effort-Associated Angina)
3. Hypertension
Included in the National Essential Medicines List and classified as Category A of National Medical Insurance; it boasts a large market scale.
Adopts a double-layer osmotic pump controlled-release formulation and is supported by national patent technology, enabling stable blood pressure reduction, minimal side effects, and greater convenience with once-daily administration.
Produced using European and American imported equipment, featuring mature manufacturing processes, large batch production, high product quality, and sufficient production capacity.
Has passed the Consistency Evaluation of Generic Drugs, ensuring its quality and therapeutic effect are consistent with the original research drug.