
Founded Ousheng Pharm. in Chengdu, starting business layout.
Relocated production base from Chengdu to Beijing, constructed the Phase I project, and completed the adjustment of company core location.

Obtained 4 Chinese patents for the core technology, as follows: ZL200610153091.8, ZL200610113725.7, ZL200610113984.X, ZL200610114125.2.

Obtained Chinese patent for the core technology: ZL200710177989.3.

Awarded the titles of "National High-Tech Enterprise" and "Zhongguancun High-Tech Enterprise", and has maintained both qualifications ever since.

Gifted the world’s first fully automatic mechanical drilling machine from ALZA Corporation (U.S.A.) for controlled-release tablet testing.

Started construction of the Phase II project of Beijing Huairou Plant officially, expanding local production.

Invested in land acquisition in Zhenjiang, Jiangsu; launched new production base planning.
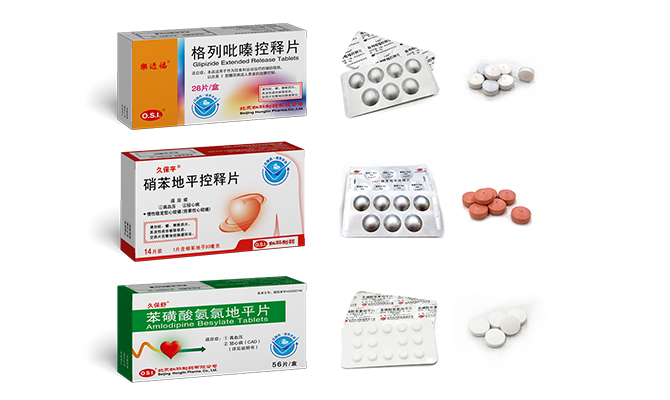
RoToFier®、JUPOPINE®、JUPOSHU® passed the Consistency Evaluation of quality and efficacy of Generic Drugs, and JUPOPINE® was officially designated as “Nifedipine Controlled-Release Tablets”

Zhenjiang plant held a groundbreaking ceremony and entered substantial construction.
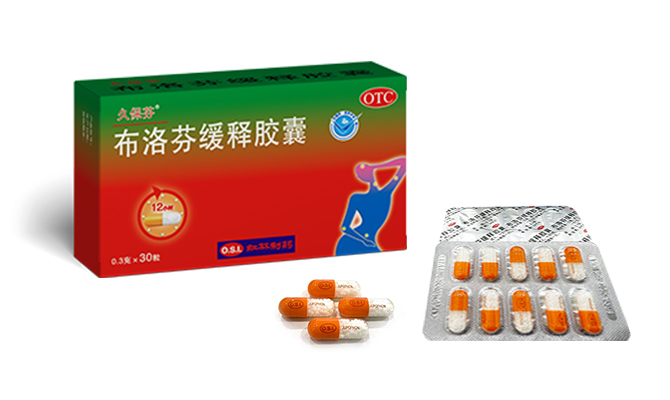
JUPOPHON® officially passed the Consistency Evaluation of quality and efficacy of Generic Drugs

Obtained a utility model patent: ZL202420848264.1.

Zhenjiang plant completed and inaugurated.

Zhenjiang Plant officially started production, with the company's large-scale capacity fully achieved.